Regioselective facile synthesis of novel isoxazole-linked glycoconjugates†
Abstract
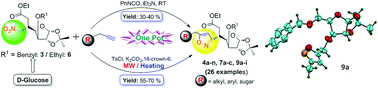
A concise and efficacious protocol for the regioselective synthesis of novel 3,5-disubstituted isoxazole-linked glycoconjugates (4, 7 and 9) via a 1,3-dipolar cycloaddition reaction between in situ generated glycosyl-β-nitrile oxide (derived from glycosyl-β-nitromethane ester 3 and 6) and various terminal alkynes bearing sugar, alkyl and aryl substituents (2a–n), has been devised. The formation of nitrile oxide during the reaction course has been supported by DFT calculations, which gave the optimized structure of the glycosyl-β-nitrile oxide ester. This one-pot methodology offers a way for utilizing D-glucose derived nitrile oxide, as a new variant in click chemistry for the synthesis of novel isoxazole-linked glycoconjugates, paving a new route for the construction of carbohydrate based scaffolds of multifaceted biological profiles.

 Please wait while we load your content...
Please wait while we load your content...