Electrochemical-driven water reduction and oxidation catalyzed by an iron(iii) complex supported by 2,3-bis(2-hydroxybenzylideneimino)-2,3-butenedinitrile†
Abstract
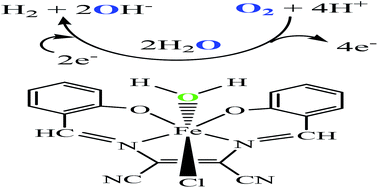
One molecular electrocatalyst for both water reduction and oxidation, based on an iron(III) complex [FeLCl(H2O)] 1, is formed by the reaction of anhydrous FeCl3 with a tetradentate ligand, 2,3-bis(2-hydroxybenzylideneimino)-2,3-butenedinitrile (H2L). Its structure has been determined by X-ray diffraction. 1 electro-catalyzes hydrogen evolution both from acetic acid and water, with a turnover frequency (TOF) of 10.25 and 808.46 moles of hydrogen per mole of catalyst per hour at an overpotential of 893 mV (DMF) and 837 mV (pH 7.0), respectively. Water oxidation occurs at an overpotential of 677 mV to give O2 with a TOF of ∼0.849 s−1.

 Please wait while we load your content...
Please wait while we load your content...