Covalent functionalization of graphene with polythiophene through a Suzuki coupling reaction
Abstract
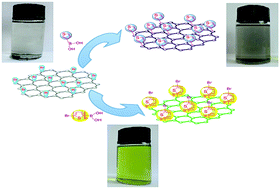
In our tentative previous work, graphene was covalently functionalized by hexylbenzene and poly(9,9-dihexylfluorene) (PF) through a Suzuki coupling reaction. In this paper, thiophene and polythiophene (PTH) were grafted to brominated graphene (Br-Gra) in the same polymeric method. The obtained conjugated system of modified graphene was characterized by various techniques, including Fourier transform-infrared (FT-IR), ultraviolet-vis (UV-vis), thermogravimetric analysis (TGA), X-ray photoelectron spectroscopy (XPS), fluorescence emission spectra, 1H-NMR spectra, and Raman spectroscopy. All the results revealed that thiophene and PTH were successfully grafted to the graphene. What's more, a colour change of their solution (Br-Gra doesn't dissolve in THF, while both thiophene grafted graphene (Gra-3TH) and PTH grafted graphene (Gra-PT) dissolve in THF, and exhibit a black and green color in THF respectively), which is caused by an electron-energy transfer between graphene and the conjugated polymer was also observed. These polymeric conjugated systems of modified graphene materials may open an interesting field for the application of graphene in photoelectric devices.

 Please wait while we load your content...
Please wait while we load your content...