Aerobic photooxidative direct asymmetric aldol reactions of benzyl alcohols using water as the solvent†
Abstract
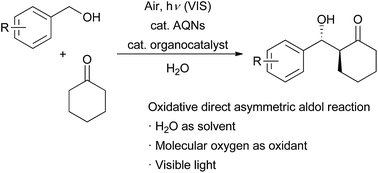
We report an aerobic photooxidative direct asymmetric aldol reaction using water as the solvent. In this reaction, primary benzyl alcohols are oxidized into benzaldehydes under an oxygen atmosphere using anthraquinone-2-sodium sulfonate monohydrate as an organophotocatalyst. Stereoselective aldol reactions then proceed using a proline-type organocatalyst.

 Please wait while we load your content...
Please wait while we load your content...