Synthesis of novel aryl dithian valeryl podophyllotoxin ester derivatives as potential antitubulin agents†
Abstract
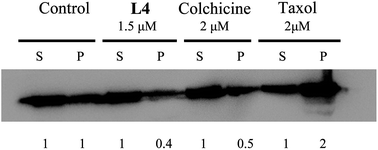
Microtubules are among the most successful targets for anticancer therapy. In this study, we described the synthesis routes of the lipoyl podophyllotoxin ester derivatives and found that they can selectively inhibit the proliferation of cancer cells without damaging the non-cancer cells. Among them, L4 showed the best antiproliferation activity with IC50 = 2.68 μM against A549 cells. This effect of L4 was similar to that of CA-4 (IC50 = 2.78 μM), a typical microtubule inhibitor, but better than podophyllotoxin (IC50 = 6.57 μM) itself. Furthermore, cell cycle analysis revealed that L4 can remarkably cause cell cycle arrest in the G2/M phase in a time- and dose-dependent manner. But the effect of L4 on apoptosis inducing was not apparent enough. Moreover, confocal microscopy and western blot analysis results indicated that L4 can perturb microtubule polymerization, thus causing tumor growth inhibition.

 Please wait while we load your content...
Please wait while we load your content...