Mechanochromism and aggregation induced emission in benzothiazole substituted tetraphenylethylenes: a structure function correlation†
Abstract
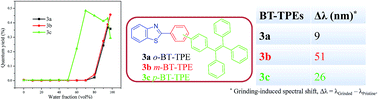
The donor–acceptor benzothiazole substituted tetraphenylethylenes (BT-TPEs) 3a–3c were designed and synthesized to examine the effect of the linkage between the BT and the TPE unit on the photophysical, aggregation induced emission (AIE) and mechanochromic properties. The syntheses of BT-TPEs 3a–3c were achieved by the Pd-catalyzed Suzuki cross-coupling reaction of bromobenzothiazoles 1a–1c with 4-(1,2,2-triphenylvinyl)phenylboronic acid pinacol ester (2). The study showed that their photophysical, AIE and mechanochromic properties are dependent on the linkage between the BT and the TPE unit (ortho, meta, and para). The meta isomer 3b shows the highest grinding induced spectral shift (51 nm) whereas the ortho isomer 3a shows the lowest grinding induced spectral shift (9 nm). The single crystal X-ray structures reveal the highly twisted conformation and tight packing in BT-TPE 3a compared to 3b. The thermogravimetric analysis of BT-TPEs shows good thermal stability. The computational study reveals the donor–acceptor nature of the BT-TPEs. The structure–function correlation indicates that the mechanochromic and aggregation induced emission (AIE) properties were dependent on the linkage between BT and TPE.

 Please wait while we load your content...
Please wait while we load your content...