Aldehyde–alkyne–amine (A3) coupling catalyzed by a highly efficient dicopper complex†
Abstract
A dicopper(I) complex, 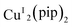 (pip = (2-picolyliminomethyl)pyrrole anion), was utilized to catalyze A3 coupling reactions, which led to the formation of propargylamines. Aldehydes, alkynes and amines with a variety of structures have been tested. A low catalyst loading of 0.4 mol% was sufficient to give good to excellent yields. The low catalyst loading, broad scope of substrate and easy preparation make this dicopper complex a useful catalyst for A3 coupling.
(pip = (2-picolyliminomethyl)pyrrole anion), was utilized to catalyze A3 coupling reactions, which led to the formation of propargylamines. Aldehydes, alkynes and amines with a variety of structures have been tested. A low catalyst loading of 0.4 mol% was sufficient to give good to excellent yields. The low catalyst loading, broad scope of substrate and easy preparation make this dicopper complex a useful catalyst for A3 coupling.


 Please wait while we load your content...
Please wait while we load your content...