Preparation of single-handed helical carbonaceous nanotubes using 3-aminophenol-formaldehyde resin†
Abstract
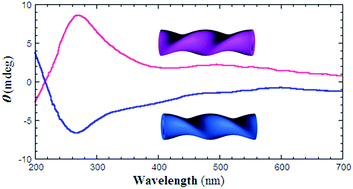
Single-handed helical 3-aminophenol-formaldehyde resin nanotubes were prepared using an external supramolecular templating method; the nanotubes were formed via the adsorption and polycondensation of 3-aminophenol-formaldehyde resin oligomers on the surface of organic self-assemblies. After carbonization at 1400 °C, single-handed helical carbonaceous nanotubes were obtained. A Raman spectrum, X-ray diffraction pattern, and high resolution transmission electron microscopy image showed that the carbon in the nanotubes was partially crystallized. Circular dichroism (CD) spectra indicated that the carbonaceous nanotubes exhibited optical activity; it was proposed that this optical activity originated from the stacking of aromatic rings. The CD spectrum was simulated using time-dependent density functional theory. When poly(n-decyl-2-methylpropylsilane) was adsorbed on the surface of the helical carbonaceous nanotubes, it tended to stack in a one-handed fashion.

 Please wait while we load your content...
Please wait while we load your content...