Dynamic redox cycle of Cu0 and Cu+ over Cu/SiO2 catalyst in ester hydrogenation†
Abstract
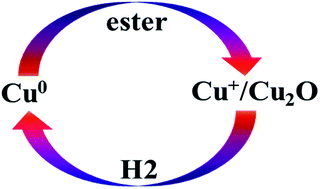
Esters (methyl acetate, ethyl acetate, dimethyl oxalate) can oxidize Cu0 of the reduced Cu/SiO2 catalyst to Cu+ and the obtained Cu+ can be reduced back to Cu0 by H2 under reaction conditions. Cu0 and Cu+ are in a dynamic cycle during ester hydrogenation. The ratio of Cu+/Cu0 in the presence of esters under reaction conditions is probably different from that in the absence of esters under characterization conditions. The effect of the dynamic cycle on the Cu+/Cu0 ratio should be taken into consideration when establishing the relationship between the Cu+/Cu0 ratio and reaction performance.

 Please wait while we load your content...
Please wait while we load your content...