Chlorosulfonic acid supported diethylamine ionic liquid catalyzed green synthesis of novel 2-mercaptonaphthalen-1-yl)methyl)-3-hydroxy-5,5-dimethylcyclohex-2-enones under neat conditions†
Abstract
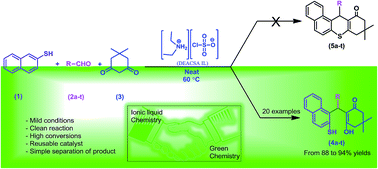
Chlorosulfonic acid supported diethylamine ionic liquid (DEACSA IL) was prepared and characterized by FT-IR, elemental analysis, NMR spectroscopy and thermogravimetric analysis. The DEACSA IL functions as an efficient heterogeneous, environmentally benign, and very active catalyst and can be recycled for several runs without any significant loss of activity with short reaction times, good to excellent yields of the desired products and high catalytic activity for the synthesis of a variety of novel 2-mercaptonaphthalen-1-yl)methyl)-3-hydroxy-5,5-dimethylcyclohex-2-enones under neat conditions. This is the first example of the condensation of naphthalene-2-thiol, aldehydes and 5,5-dimethylcyclohexane-1,3-dione to provide a novel series of thiol derivatives, which were characterized by 2D-NMR data.
- This article is part of the themed collection: Ionic Liquids: Editors collection for RSC Advances

 Please wait while we load your content...
Please wait while we load your content...