Substituted group directed assembly of zinc coordination compounds based on bifunctional ligands, from mono, di to tristetrazole–carboxylate†
Abstract
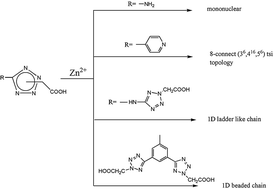
Four different tetrazole–carboxylate ligands, monotetrazole–carboxylate Hatza (Hatza = 5-aminotetrazole-1-acetic acid), Hpytza (Hpytza = 5-(4-pyridyl)tetrazole-2-acetic acid), ditetrazole–carboxylate H2datza (H2datza = N,N-di(tetrazol-5-yl)amine-N2,N2′-diacetic acid) and tristetrazole–carboxylate, H3tzpha (H3tzpha = 1,3,5-tris(tetrazol-5-yl)benzene-N2,N2′,N2′′-trisacetic acid) have been chosen to be reacted with zinc salts, resulting in the formation of four novel compounds, [Zn(atza)2(H2O)4] (1), [Zn(pytza)2] (2), [Zn(datza)(H2O)2]·3H2O (3) and [Zn3(tzpha)2(H2O)12]·MeOH·EtOH·4H2O (4), whose structures are controlled by not only the number and different coordination modes of the tetrazole–carboxylate but also the complementary hydrogen bonds. These compounds have been characterized by elemental analysis, IR and single crystal X-ray diffraction. Compound 1 displays a simple zero dimensional mononuclear structure, 2 shows a classic 3D 8-connect (36, 416, 56) tsi network topology, 3 features a 1D ladder-like chain while 4 is a 1D beaded chain. Furthermore, the luminescence properties investigated at room temperature in the solid state show excellent ligand-centered luminescence.

 Please wait while we load your content...
Please wait while we load your content...