Benzyl bromides as aroyl surrogates in substrate directed Pd catalysed o-aroylation†
Abstract
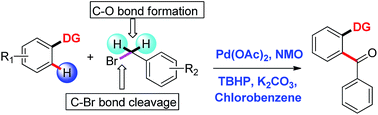
An oxidative cross-coupling between directing substrates and benzyl bromides via the combined effect of oxidants TBHP and NMO, catalysed by Pd(II) has been investigated. Benzyl bromides served as efficient aroyl surrogates in this substrate directed C–H functionalisation. The in situ generated benzaldehyde originating from benzyl bromide is the active aroylating source.

 Please wait while we load your content...
Please wait while we load your content...