TsOH-mediated reaction of aziridinofullerene with diols for the preparation of fullerene-fused dioxygenated ring compounds†
Abstract
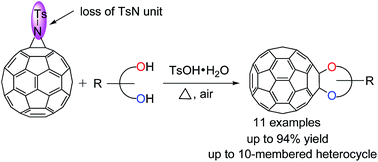
The double nucleophilic substitution reaction of aziridinofullerene with diols in the presence of p-toluenesulfonic acid affords a series of rare fullerene-fused dioxygenated ring compounds with six- to ten-membered heterocycles. A possible reaction mechanism for product formation is proposed.

 Please wait while we load your content...
Please wait while we load your content...