Adsorption of herbicide 2-(2,4-dichlorophenoxy)propanoic acid by electrochemically generated aluminum hydroxides: an alternative to chemical dosing
Abstract
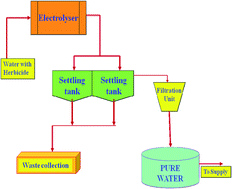
This research article presents an in situ electrosynthesis of aluminum hydroxides by anodic dissolution of sacrificial aluminum anode and their application towards the adsorption of herbicide 2-(2,4-dichlorophenoxy)propanoic acid (2,4-DP) from aqueous solution. Different sacrificial anode material like iron, magnesium, zinc and aluminum are tested and stainless steel is used as the cathode. The optimization of different experimental parameters like current density, pH, temperature and inter-electrode distance on the adsorption of 2,4-DP was carried out. The results showed that the maximum removal efficiency of 93.0% was achieved with aluminum as sacrificial anode at a current density of 0.10 A dm−2 and pH of 7.0. The adsorption of 2,4-DP preferably followed the Langmuir adsorption isotherm. The adsorption kinetic studies showed that the adsorption of 2,4-DP was best described using the second-order kinetic model. Thermodynamic parameters indicates that the adsorption of 2,4-DP on aluminum hydroxides was feasible, spontaneous and endothermic.

 Please wait while we load your content...
Please wait while we load your content...