Effect of temperature on the surface free energy and acid–base properties of Gabapentin and Pregabalin drugs − a comparative study
Abstract
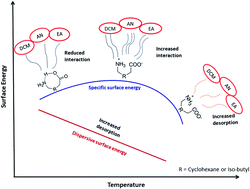
The surface energetics of Gabapentin (GBP) [2-[1-(aminomethyl) cyclohexyl] acetic acid] and Pregabalin (PGB) [(S)-3-(aminomethyl)-5-methylhexanoic acid] were studied using a surface energy analyzer (SEA); a new-generation inverse gas chromatography technique, in the temperature range of 298.15–323.15 K. The Lifshitz–van der Waals dispersive (γds) component of the surface energy was calculated using Schultz and Dorris-Gray methods. The specific free energy of adsorption (ΔGspa) and specific component of surface free energy (γsps) were determined using Schultz and Polarization methods. For both the drugs, the γds component of the surface energy was found to decrease with the increase in temperature. The γds component of the surface energy obtained for GBP and PGB surfaces suggested that GBP is slightly more energetically active than PGB. However, the PGB surface showed slightly higher γsps implying its more polar nature as compared to GBP. These drugs with different structures but identical functional groups (–NH2 and –COOH), were found to have a higher surface Lewis base parameter, (Kb), indicating predominately basic surfaces. Furthermore, the temperature dependence of the Lewis acid–base parameters for these drugs was attributed to the disruption of intramolecular hydrogen bonding at higher temperatures.

 Please wait while we load your content...
Please wait while we load your content...