Mechanism and selectivity for zinc-mediated cycloaddition of azides with alkynes: a computational study†
Abstract
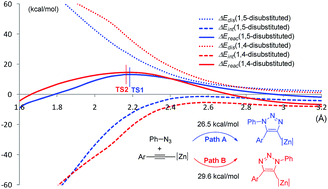
Density functional theory (DFT) method B3LYP with a dispersion term (B3LYP-D3BJ) has been used to clarify the regioselectivity of zinc mediated 1,3-dipolar cycloaddition of azides and alkynes. Computational results indicate that the dipolar cycloaddition takes place via a concerted five-membered-ring transition state, leading to a 1,5-disubstituted 1,2,3-triazole product, which is consistent with the experiment reported by Greaney's group. The coordination of imidazole ligand to zinc is reversible, and the regioselectivity is irrelevant to the coordination of imidazole ligand. Moreover, substituent effect of alkynes has also been studied. Finally, distortion–interaction analysis along the reaction pathways and frontier molecular orbital theory are used to explain the reactivity and 1,5-regioselectivity.

 Please wait while we load your content...
Please wait while we load your content...