A chromium oxide coated nickel/yttria stabilized zirconia electrode with a heterojunction interface for use in electrochemical methane reforming†
Abstract
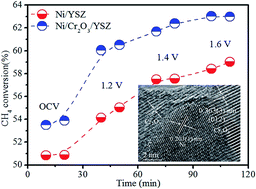
This work investigated the use of nickel/yttria stabilized zirconia (Ni/YSZ) coated in situ with chromium oxide (Cr2O3) for electrochemical methane (CH4) reforming in solid oxide electrolysers. Combined analysis using X-ray diffraction spectroscopy, transmission electron microscopy, scanning electron microscopy and X-ray photoelectron spectroscopy confirmed the formation of Cr2O3 on the Ni surface with a heterojunction interface formed by reducing nickel chromite (NiCr2O4) to a core–shell structure. The electrical properties of the Cr2O3 coated Ni/YSZ were investigated and correlated to the electrochemical performance. Significant improvements of electrode activity were achieved with Cr2O3 coated Ni/YSZ in contrast to traditional Ni/YSZ in a CH4 atmosphere. Strong carbon deposition resistance was also observed in a methane–carbon dioxide (CH4–CO2; 1 : 1) atmosphere at 800 °C. Significant enhancement in electrochemical CH4–CO2 reforming was successfully achieved in oxide ion conducting electrolysers with Cr2O3 coated Ni/YSZ cathodes.

 Please wait while we load your content...
Please wait while we load your content...