Bifurcated hydrogen bonding mediated planar 9,10-anthraquinone dyes: synthesis, structure and properties†
Abstract
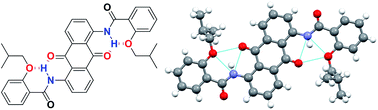
By acylation of mono- and diamino-9,10-anthraquinones with o-alkoxylbenzene carbonyl chloride or o-alkoxylnaphthalene carbonyl chloride, a series of planar 9,10-anthraquinone dyes were designed and synthesized. Because of the formation of bifurcated hydrogen bonds, these dyes adopted planar conformations, which were exemplified by the crystal structure of one dye. The UV-vis absorption spectra and FL (fluorescence) spectra of the dyes were also recorded. The extent of acylation and the positions of the amino and/or amide groups substantially affected the dyes' properties.

 Please wait while we load your content...
Please wait while we load your content...