Identification of competitive inhibitors for bovine serum albumin from dynamic combinatorial libraries containing a bienzyme system†
Abstract
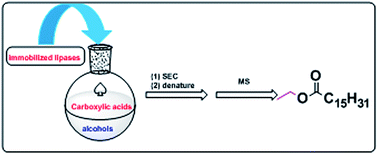
Three dynamic combinatorial libraries (DCLs) have been generated by using esterification, combined with a protocol based on size-exclusion chromatography (SEC) and HRMS. Compared with sulfuric acid and water-soluble lipase, the immobilized lipase could be recycled successfully. A new inhibitor towards bovine serum albumin (BSA) was discovered by the SEC-HRMS protocol. The binding of the new binder with BSA was investigated at different temperatures by fluorescence. The association constants K were determined by Stern–Volmer equation. The thermodynamic parameters were calculated according to the Van't Hoff equation. On the basis of the thermodynamic results, it was considered that the new compound was bound to BSA mainly by hydrophobic interaction.

 Please wait while we load your content...
Please wait while we load your content...