Enhanced removal of fluoride by tea waste supported hydrous aluminium oxide nanoparticles: anionic polyacrylamide mediated aluminium assembly and adsorption mechanism†
Abstract
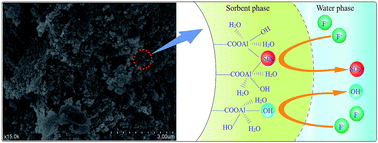
A novel and low-cost biosorbent of tea waste supported hydrous aluminium oxide (Tea–APAM–Al) was prepared with help of anionic polyacrylamide (APAM) for highly efficient defluoridation of drinking water. Batch adsorption studies were carried out by varying the adsorbent dosage, initial fluoride concentration, contact time, initial pH, and presence of co-existing ions to evaluate the efficiency of fluoride removal. It was found that Tea–APAM–Al performed well over a considerably wide pH range, from 4.0–9.0. With the exception of bicarbonate, other co-existing ions (nitrate, chloride and sulphate) did not have a significant effect on the defluoridation process. The adsorption process could be described by the Lagergren pseudo-second-order kinetic model. Adsorption data could be fitted by the Langmuir isotherm model and the maximum fluoride adsorption capacity for Tea–APAM–Al was 42.14 mg g−1. The results from SEM, EDS, XRD, FTIR and XPS studies showed that the fluoride adsorption mechanism likely involved hydroxyl and sulfate ion exchange with fluoride. Moreover, fluoride anion exchange with sulfate ions was the main mechanism for fluoride adsorption at low initial fluoride concentration.

 Please wait while we load your content...
Please wait while we load your content...