Continuous catalytic upgrading of fast pyrolysis oil using iron oxides in red mud†
Abstract
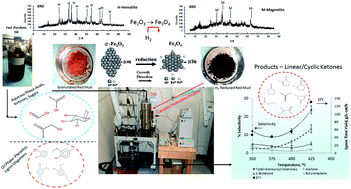
A catalyst composed primarily of magnetite was prepared from red mud, via H2 reduction at 300 °C, which significantly increased the surface area. Ammonia and CO2 temperature programmed desorption indicated both acid and base active sites. Continuous reaction studies conducted with individual compounds, mixtures of model compounds, and water extracted fast pyrolysis oil indicated that acetone was the primary product from acetic acid, and acetone and 2-butanone from acetol. Levoglucosan went down the same pathway, since it formed acetic acid, formic acid, and acetol. Total conversion and yields approached 100% and 22 mol% ketones at 400 °C and a W/F of 6 h for a model mixture and 15–20 mol% ketones at W/F 1.4–4 h and 400–425 °C using water extracted oil. Space time yields approached 60 g ketones per L-cat per h for the model mixture and 120 g per L-cat per h for a commercial oil. The catalyst simultaneously reduced acidity, allowed recovery of carbon, and generated upgradable intermediates from the aqueous fraction of fast pyrolysis oil in a “continuous” process.

 Please wait while we load your content...
Please wait while we load your content...