Synthesis, glycosylation and NMR characterization of linear peracetylated d-galactose glycopolymers†
Abstract
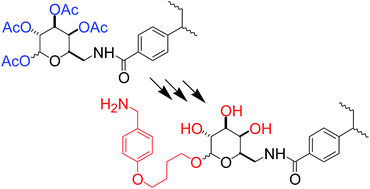
The glycosylation of D-galactose glycopolymers to produce functionalized polymers expected to be bioactive as substrates or inhibitors of enzymes of the copper amine oxidase class (CAOs, EC 1.4.3.6), attained previously with an unpractical, but significant process requesting a large excess of glycosyl acceptor, has now been achieved with a method based on the use of peracetylated D-galactose moieties, in the presence of SnCl4 or BF3·Et2O as promoters, which employs glycosyl donors in nearly stoichiometric ratio with the desired glycosyl acceptor. After the synthesis of an appropriate model molecule acting as a guide, the new method was set up with extensive NMR investigations on the same model molecule and soluble polymeric reagents and products, performing a full rationalization of the chemical behavior of the various α and β pyranose and furanose forms of D-galactose residues. Glycosylated systems containing functionalities protected with trifluoroacetyl and acetyl groups were fully deprotected in one step through a reduction procedure with NaBH4.

 Please wait while we load your content...
Please wait while we load your content...