The influence of various LiF concentrations on the cathodic electrochemical behavior at the tungsten electrode in Na3AlF6–Al2O3 molten salt
Abstract
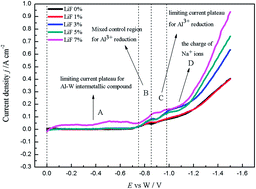
The electrochemical deposition process of Al metal at the tungsten electrode in the melts of Na3AlF6–Al2O3 with various LiF concentrations was investigated at 1253 K by various electrochemical techniques. With the analysis of the potentiodynamic cathodic polarization curves, it can be demonstrated that LiF has an effect of increasing the conductivity of the molten salt. From the analysis of the potentiostatic electrolysis and chronopotentiometry curves, it can be deduced that the deposition potential of Na metal is more positive with the increase of LiF concentration. Spontaneous dissolution of the cathodic products occurs for the polarized electrode which is shown by the measurements of open-circuit chronopotentiometry. These show that the scanning time taken for the potential to reach zero is prolonged and the thickness of the reduced species to be dissolved increases with the increase of LiF concentration. Furthermore, it can be deduced that the Al activity also increases with the increase of LiF concentration. On the other hand, the deposited Al is more easily dissolved into the electrolyte and the physical dissolution is more intense with the increase of LiF concentration.

 Please wait while we load your content...
Please wait while we load your content...