Influence of Mn incorporation on the supercapacitive properties of hybrid CuO/Cu(OH)2 electrodes†
Abstract
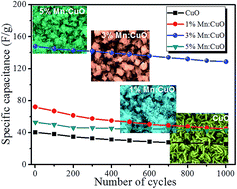
Here, we are presenting the effect of Mn doping on the supercapacitive properties of CuO/Cu(OH)2 hybrid electrodes. Briefly, Mn doped CuO/Cu(OH)2 (Mn:CuO/Cu(OH)2) thin films have been synthesized by a successive ionic layer adsorption and reaction (SILAR) method which are further characterized by different physiochemical techniques. Our results revealed the formation of hybrid CuO/Cu(OH)2 thin films with significant morphological deviation through Mn doping. Moreover, considerable positive effect of Mn doping on the electrochemical properties of hybrid CuO/Cu(OH)2 electrodes have been witnessed. Later, the results suggest that at 3% Mn doping in CuO/Cu(OH)2 electrodes with nanoflower-like nanostructures exhibits the highest specific capacitance. The maximum specific capacitance achieved for a 3% Mn:CuO/Cu(OH)2 hybrid electrode is 600 F g−1 at 5 mV s−1 in 1 M Na2SO4 electrolyte. Additionally, a Ragone plot confirms the potential of the Mn:CuO/Cu(OH)2 hybrid electrode for electrical energy storage applications.

 Please wait while we load your content...
Please wait while we load your content...