Adsorption of gaseous elemental mercury with activated carbon impregnated with ferric chloride
Abstract
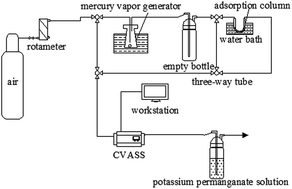
Fe-based modified activated carbon prepared by impregnation was used for adsorbents in Hg0 purification. The influence of carriers, active components, FeCl3 solution concentration and calcination temperature, were investigated for Hg0 adsorption purification. The effects of the preparation conditions on the adsorbent were characterized by N2 adsorption–desorption isotherms, scanning electron microscopy (SEM), X-ray photoelectron spectroscopy (XPS), X-ray diffraction (XRD) and (FTIR). The samples modified with FeCl3 showed an obvious performance for Hg0 removal, because the chlorine in metal chlorides acts as an oxidant that promotes the conversion of elemental mercury (Hg0) into its oxidized form (Hg2+). The mechanisms of mercury adsorption onto the Cl-impregnated activated carbon were proposed. The optimal conditions for adsorptive purification are an Fe3+ concentration of 0.15 mol L−1, calcination temperature of 300 °C at an adsorption temperature of 40 °C and carrier gas flow rate of 300 mL min−1.

 Please wait while we load your content...
Please wait while we load your content...