Photodegradation of Rhodamine B over Ag modified ferroelectric BaTiO3 under simulated solar light: pathways and mechanism†
Abstract
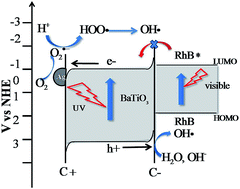
The use of semiconductors with a ‘built in’ bias has now become of interest for a growing number of photoactive applications. Using a combination of spectroscopic techniques, gas chromatography in association with mass spectroscopy and NMR, we show that a sample of ferroelectric BaTiO3 decorated with nanostructured Ag denatures a standard dye molecule (Rhodamine B) via a photocatalytic oxidation mechanism. The photosensitized oxidation was inhibited due to band bending induced by ferroelectric polarisation. In the Ag–BaTiO3 system we find a slight hypsochromic wavelength shift during the initial stages of degradation (only 3 nm before 80% degradation percentage) and associate this shift with the cleavage of the chromophore structure which pre-empted deethylation. This shift in maximum absorption of the dye molecule did not occur until the later stages of molecule fragmentation. Our major identifiable breakdown intermediate was benzoic acid. A lack of other identifiable fragments during the breakdown of the dye is associated with retention of these fragments on the catalyst as full mineralisation of the dye liberates CO2.

 Please wait while we load your content...
Please wait while we load your content...