Chaetosemins A–E, new chromones isolated from an Ascomycete Chaetomium seminudum and their biological activities†
Abstract
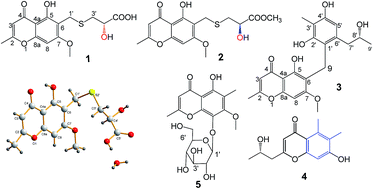
Fifteen aromatic polyketide metabolites, including four new chromones, chaetosemins B–E (2–5), with compound 4 bearing a new skeleton, and two new natural products, chaetosemin A (1) and (+)-(S)-chaetoquadrin J (14), together with nine known ones (6–13, 15), were isolated from the organic extract of a solid fermented culture of an Ascomycete filamentous fungus, Chaetomium seminudum. The structures of 1–5 were determined by spectroscopic analysis, and the absolute configuration of chaetosemin A (1) was confirmed by X-ray crystallography, whereas the stereochemistry of 1–4 and 14 was assigned by comparison of their specific optical rotations with reported data for structurally related compounds. All compounds were isolated for the first time from Chaetomium seminudum. Compounds 1 and 2 consisted of L-cysteine and D-cysteine units, respectively. Most of them were evaluated for in vitro antifungal activities against some phytopathogenic fungi. Among them, chaetosemin B (2) was the most active against Magnaporthe oryzae and Gibberella saubinettii with MICs of 6.25 and 12.5 μM, respectively. In addition, chaetosemin C (3) showed antioxidant activity with 50.7% DPPH free radical scavenging activity at 50 μM, and chaetoquadrin J (14) displayed weak inhibition of soluble epoxide hydrolase (sEH).

 Please wait while we load your content...
Please wait while we load your content...