A powerful tool for acid catalyzed organic addition and substitution reactions†
Abstract
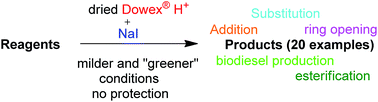
A novel green chemistry tool for acid catalyzed reactions has been developed. The multipurpose tool is based on the ability of dry solid materials to donate protons (H+) to starting materials combined with the simultaneous use of a nucleophile (e.g. NaI). The methods enable the following reactions to be conducted at 20–50 °C: selective addition of iodine or alcohols to more substituted carbon in R2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2 systems (R ≠ H), esterification reactions, e.g. free fatty acids with methanol, and at higher temperatures, (60–100 °C): esterification of free fatty acids with hindered alcohols (isopropanol), addition of iodine to C
CH2 systems (R ≠ H), esterification reactions, e.g. free fatty acids with methanol, and at higher temperatures, (60–100 °C): esterification of free fatty acids with hindered alcohols (isopropanol), addition of iodine to C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C bonds, opening of oxygen(s) containing heterocyclic rings, selective substitution of primary OH groups to iodine in the presence of other functional groups or secondary alcohol groups, esterification of alcohols with nitriles (R–CN), transesterification of fatty acid triglycerides to biodiesel and selective derivatization of primary hydroxyl groups (–CH2OH) over secondary moieties of sugars without any protection. Most of the reactions were also performed by a re-used Dowex® cation exchange resin.
C bonds, opening of oxygen(s) containing heterocyclic rings, selective substitution of primary OH groups to iodine in the presence of other functional groups or secondary alcohol groups, esterification of alcohols with nitriles (R–CN), transesterification of fatty acid triglycerides to biodiesel and selective derivatization of primary hydroxyl groups (–CH2OH) over secondary moieties of sugars without any protection. Most of the reactions were also performed by a re-used Dowex® cation exchange resin.

 Please wait while we load your content...
Please wait while we load your content...