Fabrication of resin supported Au–Pd bimetallic nanoparticle composite to efficiently remove chloramphenicol from water
Abstract
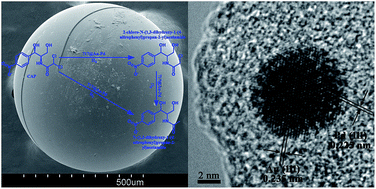
The evolution of antibiotic resistance and the potential impact on human health of chloramphenicol (CAP) have made it an environmental pollutant requiring urgent action. In this study, Au–Pd bimetallic nanoparticles (BNPs) were first synthesized and then successfully loaded on Amberlite 717 to form an Amberlite 717 supported Au–Pd BNP catalytic system (717@Au–Pd) with the mass fraction of Au–Pd at about 4.5%. The as-synthesized catalytic system was used to degrade CAP in water under a H2 atmosphere at room temperature. When 0.5 g of 717@Au–Pd was added into the CAP solution (50 mg L−1, 50 mL, pH 7), about 60% of CAP was absorbed on the 717@Au–Pd in the first 10 h and then all of CAP can be completely removed in the following 3 h under a H2 atmosphere. The degradation process of the reaction can be fitted with a first-order kinetics equation with the kinetics constant of 4.3 h−1 ± 0.009. The degradation products and mechanism were studied using LC/MSD Trap-XCT. The results showed that CAP was removed by the 717@Au–Pd via cleaving the carbon–halogen bond of CAP while keeping the nitro-group unaffected and this made the degradation products less environmentally toxic. The recycled experiments showed that the removal rate of CAP can still be maintained at 99% even after 5 cycles. The study indicated that 717@Au–Pd is a promising catalyst for removing environmental pollutants such as CAP containing carbon–halogen bonds.

 Please wait while we load your content...
Please wait while we load your content...