Synergistically thermodynamic and kinetic tailoring of the hydrogen desorption properties of MgH2 by co-addition of AlH3 and CeF3†
Abstract
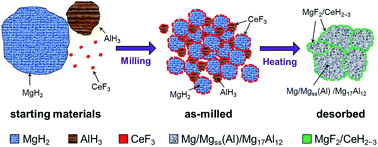
MgH2 possesses a high hydrogen capacity and excellent reversibility. However, the high thermal stability and slow sorption kinetics retard its practical application as an on-board hydrogen storage material. In this work, AlH3 and CeF3 were introduced into Mg-based materials for the purpose of improving both the thermodynamic and the kinetic properties of MgH2. DSC-TG analysis shows that the onset hydrogen desorption temperature of MgH2 can be synergistically reduced by 86 °C through the co-addition of 0.25AlH3 and 0.01CeF3. Isothermal desorption measurements demonstrate that the co-addition of AlH3 and CeF3 significantly enhances the hydrogen desorption kinetics of MgH2 with the absence of the induction period in the initial stage and the acceleration of the hydrogen desorption process. In addition, this co-doped MgH2 shows very good cycling stability at 300 °C with a 1 h capacity of 3.5 wt% and a 3 h capacity of 4.5 wt%. Structural analysis by XRD measurements indicates that during the hydrogen desorption process, MgH2 may react with Al (generated from the in situ decomposition of AlH3) to form Mg solid solution and Mg17Al12, which contribute to the thermodynamic improvement of the Mg-based material. In addition, MgH2 may also react with CeF3 to form MgF2 and CeH2–3, which act both as hydrogen diffusion gateways and as an impediment to the grain growth of MgH2 during hydrogen sorption cycling, thus improving the hydrogen desorption kinetics and the cycling stability of MgH2. Finally, it was found that the presence of AlH3 kinetically helps CeF3 to exert its positive effect on the hydrogen desorption properties of MgH2. This work provides a method for simultaneously tailoring the thermodynamic and kinetic properties of MgH2 by the synergistic addition of metal hydride and rare earth fluoride.

 Please wait while we load your content...
Please wait while we load your content...