A green electrochemical method for the synthesis of new N,N′-diphenylbenzene-1,4-diamine derivatives†
Abstract
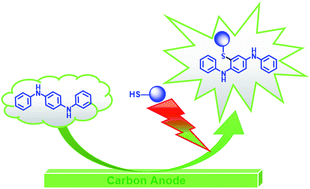
A green method for the synthesis of new organosulfur derivatives of N,N′-diphenylbenzene-1,4-diamine (2a–e) based on the Michael reaction of electrochemically generated N,N′-diphenyl-p-quinonediimine with 2-mercaptopyridine, 1H-1,2,4-triazole-3-thiol, 1-phenyl-1H-tetrazole-5-thiol, 2-mercaptobenzoxazole and 2-mercaptobenzothiazole as nucleophiles in a water/ethanol (25/75, v/v) mixture is described. The thioethers (2a–e) were synthesized in high yields, without toxic reagents and solvents at a carbon electrode using an environmentally friendly method with high atom economy.

 Please wait while we load your content...
Please wait while we load your content...