Solvent-free heteropolyacid-catalyzed glycerol ketalization at room temperature
Abstract
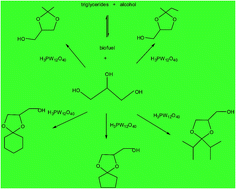
Currently, glycerol has been produced in large amounts as a biodiesel co-product. Therefore, developing processes to convert it into more valuable chemicals has attracted significant attention. Glycerol ketals are compounds useful as synthesis intermediates, fragrance ingredients, and mainly bioadditives for diesel and gasoline, and have been produced from reactions catalyzed by mineral acids. In this work, we assessed the activity of H3PW12O40 heteropolyacid on glycerol ketalization with different ketones at room temperature and in the absence of an auxiliary solvent. The effects of the principal reaction parameters such as the reactant stoichiometry, catalyst concentration, reaction temperature, and type of carbonylic reactant were investigated. H3PW12O40 heteropolyacid was much more active than other Brønsted acid catalysts evaluated (i.e. H2SO4, p-toluenesulfonic acid, H3PMo12O40 or H4SiW12O40) and exhibited high selectivity toward five-membered (1,3-dioxolane) cyclic ketals. Although homogeneous, the heteropolyacid catalyst could be recovered and reused without a loss of activity.

 Please wait while we load your content...
Please wait while we load your content...