Adsorption of fluoride on Mg/Fe layered double hydroxides material prepared via hydrothermal process
Abstract
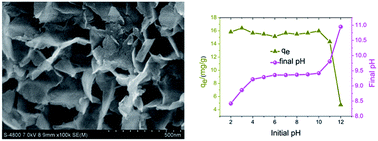
A nitrate containing Mg/Fe layered double hydroxides (Mg/Fe-LDHs) material was prepared via hydrothermal process and the calcination product (Mg/Fe-LDHO) was used as adsorbent to remove fluoride from aqueous solution. Scanning electron microscopy (SEM), X-ray diffraction (XRD), Fourier transform-infrared spectroscopy (FT-IR), thermogravimetric analysis (TGA) and pHpzc analysis were used to characterize the samples. Inductively coupled plasma-atomic emission spectrometry (ICP-AES) was employed to analyse the dissolution of the metal ions from the synthesized adsorbent and commercial activated alumina respectively. Compared with commercial activated alumina, the synthesized adsorbent entirely avoided the potential risk of alumina and had a lower leakage of metal ions. A series of batch experiments were performed to investigate the effects of various experimental parameters, such as calcination temperature, adsorbent dosage, fluoride concentration, initial pH, contact time, adsorption temperature and co-existing anions. The results showed that 270 °C was the optimal calcination temperature. The adsorption rapidly occurred in the initial 45 min and adsorption equilibrium was established within 2.5 h. Maximum desorption of 97.2% was achieved in the fluoride desorption studies at different pH. The adsorption data were well described by the pseudo-second-order kinetics model and the Langmuir isotherm model, and the adsorption capacity calculated by Langmuir equation was 28.65 mg g−1. In addition, the effects of co-existing anions on the adsorption capacity declined with the following order: PO43− > CO32− > SO42− > NO3− > Cl−. It can be concluded that the synthetic adsorbent in this study is a promising material for fluoride removal from solutions.

 Please wait while we load your content...
Please wait while we load your content...