Porous hexagonal cobalt oxyhydroxide sheets with attached nickel hydroxide nanoparticles as electrode materials for electrochemical supercapacitors
Abstract
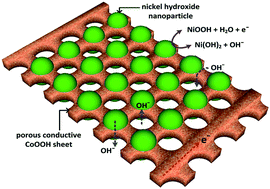
Ni(OH)2 nanoparticles were self-assembled on porous hexagonal CoOOH sheets by a simple cathodic electrophoresis in CoOOH suspension with nickel nitrate additive. A significant shift of redox peaks in the Ni(OH)2/CoOOH composite electrodes compared with the bare Ni(OH)2 electrode indicated the formation of mixed Co–Ni hydroxide layers. The amount of Ni(OH)2 nanoparticles on the porous CoOOH sheet dominated the capacitive behavior of the composite electrodes. The Ni(OH)2/CoOOH electrode with 20 wt% of Ni(OH)2 prepared in 1 mM nickel nitrate showed the highest specific capacitance and good cycle-life stability, its specific capacitance discharged at 1 A g−1 could reach as high as 1537 F g−1 much higher than that of bare CoOOH (52 F g−1) and Ni(OH)2 (785 F g−1) electrodes. A noticeable improvement in capacitive behavior resulted from the porous conductive CoOOH sheets that facilitated the transport of electron and electrolyte.

 Please wait while we load your content...
Please wait while we load your content...