Kinetics and mechanism study of direct ozonation organics in aqueous solution
Abstract
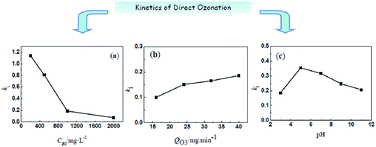
In this study, the kinetics and mechanism of direct ozonation organics in aqueous solution were explored. Phenoxyacetic acid was selected as the model pollutant and ozonation experiments were performed in a bubble batch reactor to determine the rate constants for the direct reaction. Two kinetic methods were used for determination of different kinetic rate constants (kapp and ki). The first group of results showed that degradation of phenoxyacetic acid followed pseudo-first-order kinetics. A simplified model was derived related to the operational parameters on phenoxyacetic acid degradation, and the apparent rate constant kapp was obtained. The reaction was proved in the slow kinetics of the gas–liquid reaction, and the kinetic constant ki was built. Influence of pH on kapp and ki, the O3 dosage, and the initial phenoxyacetic acid concentration were carefully analyzed.

 Please wait while we load your content...
Please wait while we load your content...