Preparation of a reduced graphene oxide hydrogel by Ni ions and its use in a supercapacitor electrode†
Abstract
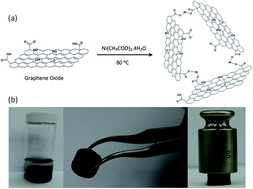
A three-dimensional (3D) reduced graphene oxide hydrogel (rGOH) was prepared by hydrothermal synthesis based on the electrostatic force and chemical reaction between graphene oxide (GO) and Ni ions in a nickel acetate solution. The Ni–rGOH fabricated in this study exhibited a highly increased surface area compared to NiO nanoparticles owing to the formation of 3D networks. After the reduction of functional groups in Ni–GOH, Ni–rGOH showed highly enhanced capacitance (351 F g−1) at a charge/discharge current density of 0.625 A g−1 and 90% capacitance retention after 1000 cycles. The energy density of Ni–rGOH was 175.5 W h kg−1 at a power density of 1.125 kW kg−1, while maintaining a high-energy density of 118 W h kg−1 at a power density of 4.5 kW kg−1.

 Please wait while we load your content...
Please wait while we load your content...