l-Proline catalyzed three-component synthesis of para-naphthoquinone–4-aza-podophyllotoxin hybrids as potent antitumor agents†
Abstract
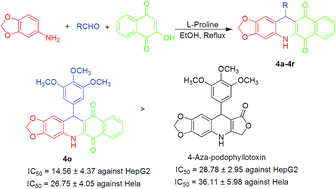
A series of novel para-naphthoquinone embodied 4-aza-podophyllotoxin hybrids, designed via a molecular hybridization approach, were synthesized in very good yields using one-pot condensation of 3,4-methylenedioxyaniline, aldehydes and 2-hydroxy-1,4-naphthoquinone in the presence of L-proline. All the synthetic derivatives were fully characterized by spectral data and evaluated for their antitumor activity on human hepatoma cells (HepG2) and the Henrietta Lacks strain of cancer cells (Hela). Among the 18 new compounds screened, 12-(3,4,5-trimethoxyphenyl)-5,10-dihydro-benzo[i][1,3]dioxolo[4,5-b]acridine-6,11-dione (4o) has pronounced activity. The results demonstrated the potential importance of molecular hybridization in the development of 4o as a potential antitumor agent.

 Please wait while we load your content...
Please wait while we load your content...