Formation and investigation of unstable complex hydrides from tetrabutylammonium fluoride, water and SO2
Abstract
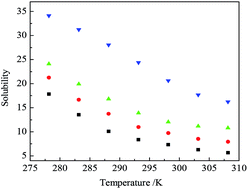
A phase separation occurred when SO2 was introduced into the binary system of tetrabutylammonium fluoride (TBAF) and water system. The mechanics of this phase separation process were studied by Fourier transform-infrared spectroscopy (FT-IR), Raman spectroscopy and Karl-Fischer titration. A complex hydrides of TBAF–SO2–H2O was formed and the TBAF and SO2 concentrated in the lower layer, and it could be released by heating the solution under reduced pressure (382.2 K, 10.1 kPa). The equilibrium solubility of SO2 in the binary system of tetrabutylammonium fluoride and water was investigated. After desorption, the mixture could be reused to absorb SO2. The effect of temperature and concentration of TBAF on the mole ratio of SO2–TBAF and H2O–TBAF of the triple hydrate was studied. The binary system of TBAF and water could absorb SO2 efficiently.

 Please wait while we load your content...
Please wait while we load your content...