Preparation of iodonium ylides: probing the fluorination of 1,3-dicarbonyl compounds with a fluoroiodane†
Abstract
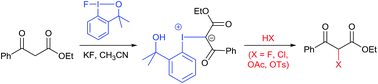
The isolation of iodonium ylide 8, from the reaction of fluoroiodane 1 with ethyl 3-oxo-3-phenylpropanoate 5 in the presence of potassium fluoride, provides strong evidence that 1,3-dicarbonyl compounds undergo an addition reaction with fluoroiodane 1 to form an iodonium intermediate which can be deprotonated to generate an iodonium ylide. In the presence of TREAT-HF, however, the iodonium intermediate reacts to form the 2-fluoro-1,3-dicarbonyl product and we propose that fluoroiodane 1 simulates electrophilic fluorination via an addition/substitution mechanism. Further evidence to support this mechanism was obtained by successfully reacting the isolated iodonium ylide 8 with TREAT-HF, hydrochloric acid, acetic acid and p-toluenesulfonic acid to form the 2-fluoro-, 2-chloro-, 2-acetyl- and 2-tosyl-1,3-ketoesters respectively.

 Please wait while we load your content...
Please wait while we load your content...