Chalcomer assembly of optical chemosensors for selective Cu2+ and Ni2+ ion recognition†
Abstract
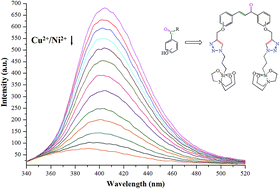
The o-, m- and p-isomeric units of chalconyl triazole-based, caged organosilicon complexes were efficiently synthesized and explored for their cationic chemosensing activities. The UV-vis spectral studies performed show considerable variations in absorption spectra and molar absorptivity constant. The recognition studies display efficient sensing for the o-isomer of chalcone-linked 1,2,3-triazole silatrane (CTSI) 1–3, which act as dual-ion fluorescent sensors towards Cu2+ and Ni2+ ions. This preference of o-isomers (CTSI 1–3) over m- and p-isomers (CTSI 4–9) in quenching is due to specific ‘fitting in’ of the coordination sphere available for ion binding. Further, the exceptional activity of CTSI 8 to exclusively sense Ni2+ ions differs from the other studied quenching response patterns, acting via a ‘turn-on’ fluorescence response. The variation of pH and temperature on the chemosensing behavior of CTSI 1–3 led us to optimize conditions for quenching studies. Moreover, competitive quenching studies confirm the feebly enhanced selectivity for Cu2+ over Ni2+ ions. Stern–Volmer constant (KSV) for all active isomers show comparative quenching response towards both cationic species. This is the first the time that organosilicon complexes are used to actively sense Cu2+ and Ni2+ ions using water as part of the solvent mixture.

 Please wait while we load your content...
Please wait while we load your content...