Indium(iii)-catalyzed tandem synthesis of 2-alkynyl-3,3-dichloropyrrolidines and their conversion to 3-chloropyrroles†
Abstract
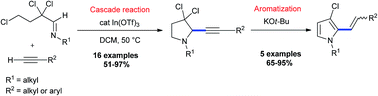
The synthetic utility of electron-deficient α,α,γ-trichloroaldimines was demonstrated by an indium(III) triflate-catalyzed cascade reaction with terminal alkynes allowing one to rapidly and selectively access 2-alkynyl-3,3-dichloropyrrolidines in good to excellent yields. The reaction proceeds in a single synthetic operation via an addition of acetylenes to α,α,γ-trichloroaldimines, followed by a spontaneous cyclization of the in situ formed trichloropropargylic amines. The dichloromethylene moiety of the aldimine acts as an activating group to accomplish this transformation under very mild conditions. A broad variety of both aryl and alkyl acetylenes, as well as primary and secondary nitrogen substituents in the imine are well tolerated. The dichloromethylene group, which is conserved in the 2-alkynylpyrrolidine enhances the synthetic value of these pyrrolidines and allowed their conversion to (E/Z)-2-alkenyl-3-chloropyrroles by a base induced monodechlorination.

 Please wait while we load your content...
Please wait while we load your content...