tert-Butyl hypochlorite mediated diastereoselective oxidative coupling: access to 1-functionalized tetrahydrocarbazoles†
Abstract
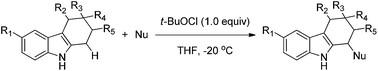
A mild and operationally simple protocol for the direct C–H functionalization of the 1-position of tetrahydrocarbazoles is reported. The diastereoselective oxidative coupling process is mediated by tert-butyl hypochlorite, and allows the successful generation of 1-functionalized tetrahydrocarbazoles with good to excellent yields and good functional group tolerance.

 Please wait while we load your content...
Please wait while we load your content...