Theoretical study of deuterium isotope effects on acid–base equilibria under ambient and hydrothermal conditions†
Abstract
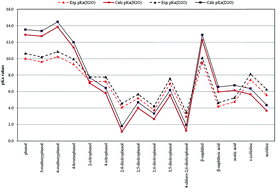
Quantum electronic structure methods are applied for the first time to the study of deuterium isotope effects (DIE) on pKa values under ambient (25 °C, 101.3 kPa) and hydrothermal (250 °C, 20.0 MPa) conditions. This work focuses on sixteen organic acids and explores several methodologies for calculating pKa values and various pKa differences in H2O and D2O under two sets of conditions. Two functionals are considered (B3LYP and BLYP) and solvent effects are accounted for by means of continuum solvation methods (PCM, CPCM, Onsager and SMD). Excellent agreement with experiment is obtained for the calculated DIE (ΔpKa = pKa(D2O) − pKa(H2O)) at the B3LYP-PCM/6-311++G(d,p) level of theory for the two sets of conditions. These values, which are almost constant for a given set of temperature and pressure conditions, are determined by the difference between the Gibbs free energies of formation of the acid and its deuterated form in each solvent. However, accurate predictions under ambient conditions can also be made from zero-point energy differences. The average calculated ΔpKa values under ambient (experimental average: 0.53) and hydrothermal conditions were 0.65 and 0.37, respectively. The mean absolute error between calculated and experimental ΔpKa values under ambient conditions was 0.11. The methodology applied is a very important tool for accurately predicting DIE on pKa values under both ambient and hydrothermal conditions, which can be used to make accurate pKa predictions in D2O.

 Please wait while we load your content...
Please wait while we load your content...