The first 4,4′-imidazolium-tagged C2-symmetric bis(oxazolines): application in the asymmetric Henry reaction†
Abstract
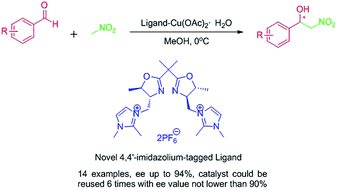
Highly efficient and recyclable imidazolium-tagged bis(oxazolines), with an imidazolium tagged onto the 4,4′-position of the box, have been designed and prepared for the first time. They have been synthesized from dimethylmalonic acid and used as chiral ligands in the copper(II)-catalyzed classic asymmetric Henry reaction between aldehydes and nitromethane. A systematic analysis of the anions showed that the best ligand was one of a medium size; the catalyst achieved a high activity and enantioselectivity as well as good recyclability, i.e., product (R)-11k was attained at 94% ee in MeOH. Moreover, the catalyst was successfully recycled six times, without an obvious loss in activity or enantioselectivity. Finally, a theoretical mechanistic study was conducted to explain the origin of the enantioselectivity and how the size of anions affects the reaction.

 Please wait while we load your content...
Please wait while we load your content...