Effective, transition metal free and selective C–F activation under mild conditions
Abstract
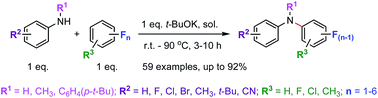
A simple and effective aromatic nucleophilic monosubstitution reaction for the synthesis of aromatic amines via selective C–F bond cleavage of various fluoroarenes (mono-, di-, tri-, tetra-, penta- and perfluorobenzene) with primary and secondary aromatic amines under transition metal free conditions is demonstrated. The reaction conditions were investigated thoroughly for a wide range of fluoroarene substrates bearing different numbers of fluorine atoms, and the results showed that the solvent and reaction temperature are very crucial for the successful substitution reactions. The established methods enabled the formation of nonfluorinated and partially fluorinated aromatic amines in good to excellent yields. Several functional groups were tolerated under the optimized conditions.

 Please wait while we load your content...
Please wait while we load your content...