Comparative study of ring-opening polymerization of l-lactide and ε-caprolactone using zirconium hexadentate bis(aminophenolate) complexes as catalysts†
Abstract
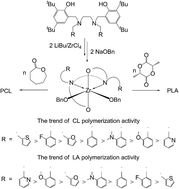
A series of zirconium bis(aminophenolate) complexes as catalysts for the ring opening polymerization of L-lactide (LA) and ε-caprolactone (CL) were investigated. Ligands bearing various chelating groups have a profound influence on the catalysis results. Among them, the thiophen-2-yl methyl group showed the greatest activity while the pyridine-2-yl methyl group showed the worst performance with regard to the rate of CL polymerization. However, the trend was reversed for the rate of LA polymerization. The kinetic results indicated a first-order dependency on [CL] and [LA]. However, the order of the catalyst concentration was different. Polymerization proceeded with second-order dependence on [LOMeZr(OBn)2] for CL but with first-order dependence on [LOMeZr(OBn)2] for LA.

 Please wait while we load your content...
Please wait while we load your content...