Dipropargyl ether bisphenol A based boron-containing polymer: synthesis, characterization and molecular dynamics simulations of the resulting pyrolysis and carbonization
Abstract
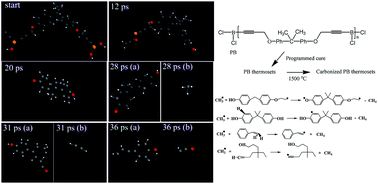
Boron-containing polymers have recently attracted worldwide attention due to their ability to affect the thermal and oxidation resistance of an in situ formed material during pyrolytic carbonization. In this work, a novel dipropargyl ether bisphenol A based boron-containing (PB) precursor polymer, which can thermally cure via propargyl groups, was synthesized. PB was characterized with Fourier transform infrared spectroscopy and nuclear magnetic resonance (1H, 11B and 13C). Thermogravimetric analysis indicated the outstanding thermo-oxidative stability of PB thermosets with the temperature of 5% weight loss of 362 °C and char yield of 56.7% at 800 °C in air, while those are 416 °C and 74.0%, respectively, in nitrogen. The time evolution of major pyrolysis products including pyrolysis mechanism of PB thermosets were examined via thermogravimetry-Fourier transform infrared spectra as well as reactive molecular dynamics (ReaxFF-MD) simulations. In addition, the carbonized structure of PB thermosets was analyzed using X-ray photoelectron spectroscopy and X-ray powder diffraction. Formation of the graphitic fragment was simulated with ReaxFF-MD. Combining the ReaxFF-MD simulation with the experimental analysis, the way that boron atoms insert into the graphitic structure was illustrated.

 Please wait while we load your content...
Please wait while we load your content...