Tailoring the dissolution rate enhancement of aminoglutethimide by functionalization of MCM-41 silica: a hydrogen bonding propensity approach†
Abstract
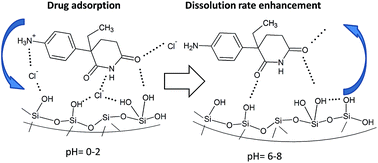
The adsorption and in vitro release properties of the poorly soluble cytostatic agent DL-aminoglutethimide (AGT) on pristine MCM-41, 3-aminopropyl and a novel N-propyl-2-sulfanylacetamide functionalized MCM-41 material were studied. The mesostructured supports and hybrid samples were characterized by small- and wide-angle X-ray diffraction, FT-IR spectroscopy, thermogravimetric and calorimetric analyses, SEM-EDX and N2 adsorption–desorption isotherms. The drug uptake was found to be strongly influenced by its ionization state and solution pH. Drug release experiments show a spectacular increase in the dissolution rate of the therapeutic agent for all hybrid samples, indicating that aminoglutethimide encapsulation into the mesopores of MCM-41-type supports is a viable strategy for dissolution rate enhancement. Using a knowledge-based logit hydrogen bonding propensity model to assess the relative strengths of drug–support supramolecular interactions, we have proposed that a Si–OH⋯Cl− AGT+ mechanism is responsible for the increased drug adsorption in acid media and release rate enhancement at physiological pH.

 Please wait while we load your content...
Please wait while we load your content...