An efficient d-glucosamine-based copper catalyst for C–X couplings and its application in the synthesis of nilotinib intermediate†
Abstract
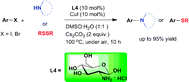
D-Glucosamine has been studied for C–N and C–S bond formations via cross-coupling reactions of nitrogen and sulfur nucleophiles with both aryl iodides and bromides. Imidazoles, benzimidazole, indole, pyrrolidine and diphenyl disulfide undergo reactions with aryl halides in the presence of 10 mol% D-glucosamine, 10 mol% CuI, and 2 equiv. of Cs2CO3 in DMSO–H2O at moderate temperature to give the corresponding products in good to excellent yields. Substrates bearing halides, free amino groups, trifluoromethyl and heterocycles were well tolerated. The high water solubility of the ligand enables easy catalyst removal. In addition, the application of this catalytic system for the synthesis of nilotinib intermediate was also successfully demonstrated using commercially available substrates.

 Please wait while we load your content...
Please wait while we load your content...