A new ion exchange adsorption mechanism between carbonate groups and fluoride ions of basic aluminum carbonate nanospheres†
Abstract
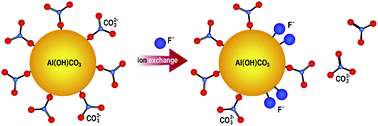
Excessive fluoride in water has serious effect on people's health and removal of it through adsorption is very effective and important. Developing adsorbents with high adsorption capacities and elucidating the adsorption mechanisms are the two aspects needed to be enhanced. Herein, we reported that basic aluminum carbonate (denoted as Al(OH)CO3) nanospheres with an abundance of carbonate groups exhibited excellent properties for removal of fluoride with maximum adsorption capacity of 59 mg g−1 in neutral solution. In particular, other common anions in water, such as Cl−, NO3−, PO43−, SiO32− and SO42−, had no significant effects on the adsorption properties of Al(OH)CO3 for fluoride. Based on the X-ray photoelectron spectroscopy (XPS) and Fourier transform infrared spectroscopy (FTIR) results, a new ion exchange mechanism involving carbonate groups and fluoride ions in solution was proposed.

 Please wait while we load your content...
Please wait while we load your content...